February 05, 2025
Understanding the Mole Concept and Its Importance in Chemistry
The mole is one of the most basic physical chemistry topics. The mole concept is something that you will come across whether in high school learning chemistry for the first time or in preparing for competitive exams like NEET. The more you understand it, the more you will be able to solve problems quickly, and it will also help you to understand chemistry as a whole. we will understand the mole concept and break it down step by step, as well as its importance in chemistry. Let’s get started!
What is the Mole Concept?
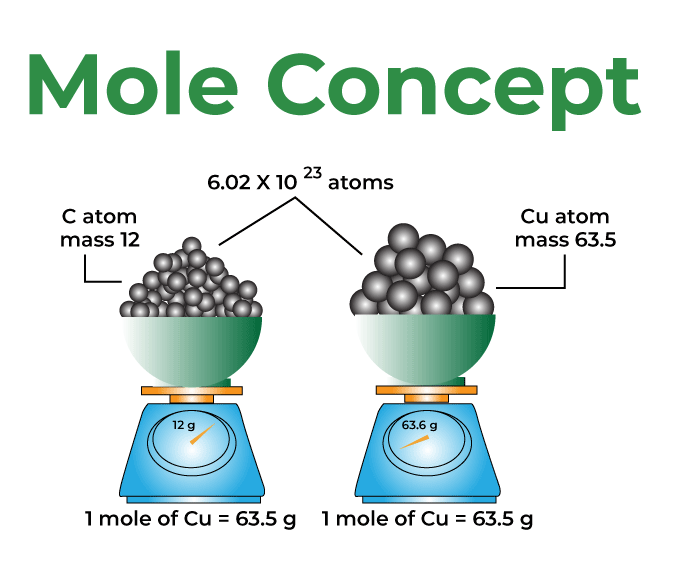
The mole is a way to count particles, atoms, molecules, or ions in a substance. When we began learning chemistry, we were told that the atomic mass of carbon was 12 grams. But how does that make sense? What makes a single atom of carbon weigh 12 grams? So, scientists came up with the idea of using a mole to resolve this.
Instead of saying the carbon atom weighs 12 grams, they said 6.023 × 10²³ carbon atoms (Avogadro’s number). If you have 6.023 x 10²³ atoms of carbon, the total mass of the atoms is 12 grams. This large number makes the idea of counting atoms more manageable. This is referred to as "one mole" of carbon.
What Does One Mole Represent?
Regardless of the substance, a mole is a specific number of particles, 6.023 × 10²³.
For example:
- The weight of 1 mole of carbon atoms is 12 grams.
- Water’s molecular mass is 18 g/mol, so 1 mole of water molecules weighs 18 grams.
- 32 grams is the weight of 1 mole of oxygen molecules.
The number of particles in one mole is constant regardless of the substance, and the mole is a very useful concept in chemistry.
Is the Number 6.023 × 10²³ Universal?
Yes! This number is universal. This is not limited to carbon. No matter if it’s carbon atoms, water molecules, or oxygen molecules, 6.023 × 10²³, is the magic number for one mole of any substance.
For example:
- The weight of 1 mole of water molecules (18 grams) is 6.023 × 10²³ molecules.
- 32 grams is the weight of 1 mole of oxygen molecules (6.023 × 10²³ molecules).
The number of particles in one mole does not depend on the substance. This universal constant makes calculations in chemistry easier and more straightforward.
Using the Mole Concept in Chemical Reactions
The mole concept is very useful when we study chemical reactions. Let’s take the reaction:
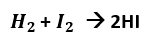
According to Gay-Lussac's Law, 1 volume of hydrogen gas reacts with 1 volume of iodine gas to yield 2 volumes of HI gas. This reaction can also be expressed in terms of moles.
- There are 2 moles of HI for every mole of hydrogen and every mole of iodine.
The mole concept is an advantage because it simplifies the calculations, and you don’t have to worry about the units that are involved, such as volumes, pressure, or temperature. You can just work out the moles from the substance involved in the reaction and ignore the rest.
Simplifying Calculations with the Mole Concept
The mole concept is very useful, especially when you want to calculate moles from the mass of a substance.
For instance, we may wish to know how many moles are in 64 grams of oxygen. For this, we divide the mass (64 grams) by the molecular weight of oxygen (32 grams per mole).
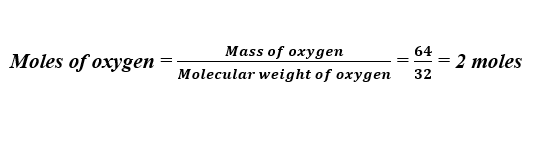
If we want to find the number of molecules in 2 moles of oxygen, we just multiply the number of moles (2) by Avogadro's number.
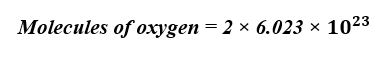
If we need the number of atoms, we multiply the number of molecules by 2 (oxygen is a diatomic molecule, so each molecule contains two atoms). Therefore, the total number of oxygen atoms will be -
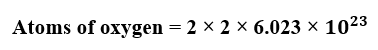
learn more about the mole concept and its application in chemistry, then watch our YouTube video explaining it in detail.
Determining Molecules and Atoms from Moles
This is where the mole concept shines. After knowing how many moles of a substance you have, it is easy to know how many molecules or atoms it contains. For example, if we have 2 moles of oxygen molecules, we can easily calculate how many molecules and atoms are involved in the case of oxygen.
Suppose we have 1 mole of water (H₂O). Then we know that it consists of 6.023 × 10²³ water molecules. To get the number of hydrogen atoms in 1 mole of water, we multiply the number of molecules by 2 (because each water molecule contains two hydrogen atoms).
The Role of the Mole Concept in Gases
Gases behave in a way that is governed by specific laws, such as Avogadro’s Law. According to Avogadro's Law, at the same temperature and pressure, the same volume of gases contains an equal number of molecules.
The volume of 1 mole of any gas at standard temperature and pressure (STP) is 22.4 litres. It is a universal constant the volume of 1 mole of any gas will always be 22.4 litres at STP.
The ideal gas equation (PV = nRT) can also be used to calculate the behaviour of gases.
- P is pressure,
- V is volume,
- n is the number of moles,
- In this case, R is the ideal gas constant,
- T is temperature.
This equation gives us an idea of gas behaviour under different conditions and how to calculate it.
Conclusion
The mole concept is a very useful tool in chemistry to simplify complex calculations and to understand chemical reactions and the behaviour of gases. Knowing how many particles are in a mole will allow you to easily calculate how many substances, molecules, or atoms are involved in a reaction. The mole concept is not only a theoretical concept but also a practical one that helps to make chemistry easier.