January 05, 2025
Understanding Risks and Controls in Nuclear Chemistry
Nuclear chemistry is the branch of chemistry that deals with nuclear reactions, isotopes properties, and the process through which the reactions occur. It consists of various sorts of nuclear reactions, fission, fusion, and radioactive decay, of different types distinguished by their properties and uses. Know more about radiation, whether it is coming from natural or manmade origin, and the most frequent isotopes being utilized in different sectors.
Radiation has known biological impacts; this implies that every dose of radiation should be quantified and controlled for acute and chronic effects. As for the safety precautions, it should be understood that radiation protection principles, individual protective measures should be used constantly while working with radioactive materials.
A good nuclear waste management plan means first defining different types of nuclear wastes and the containment and disposal of the same.
Risk management in nuclear chemistry is expressed as the identification of the risks, the analysis of their consequences, and the management of the risks. Nuclear chemistry has immense importance in present day medicine, industry, and research.
These challenges indicate that it is possible to take advantage of nuclear chemistry without jeopardizing the general populace, and the surrounding environment, provided that the risks attached to nuclear chemistry are well managed and understood.
The Introduction
- Nuclear chemistry is the branch of chemistry involved with nuclear reactions, and the chemical uses of nuclear phenomena.
- Nuclear chemistry deals with aspects like radioactive decay, nuclear fission, and fusion as well as isotopic labeling that have applications in the generation of nuclear power, in diagnostic imaging and treatments, and in industries.
- Nuclear chemistry is not limited to a plain study of atomic theory but includes atomic energy and the processes of radiation. This incorporates the examination of both stable and unstable isotopes, and explaining how nuclei of the isotopes discharge energy in the process of decay.
- Nuclear chemists dedicate their strive to make use of this energy for many purposes including scientific developments in the society such as medicine purposely deploying isotopic techniques for imaging and treatment as well as employing energy from nuclear reactions for generation of a significant portion of the electricity used globally.
Key Aspects of Nuclear Reactions
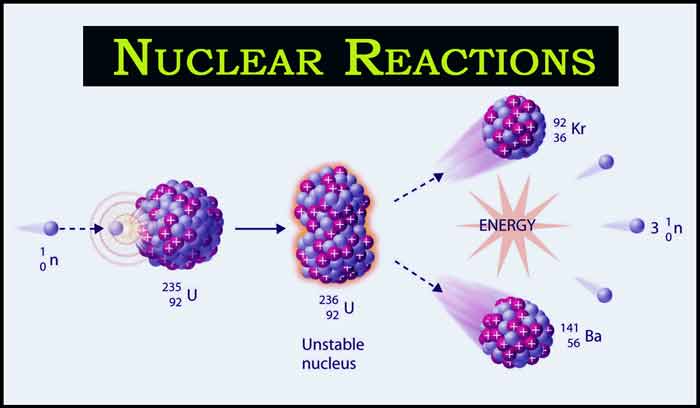
1. Fission: Nuclear fission is a form of nuclear reaction where the nucleus of an atom is divided into two or more smaller particles or nucleons accompanied with two or three neutrons as well as release of a large quantity of energy. This process can occur at intervals without any external stimuli, however, mostly the process is stimulated by the interaction of an element with a neutron, usually uranium-235 or plutonium- 239. In the fission process, a large quantity of energy is produced whose origin is revealed by the classical equation E=mc2 where E is energy, m is mass and c is velocity of light.
When it occurs, the nuclear fission process is generally characterized by the spitting off of two fission fragments with unequal mass the combined mass of the fragments is less than the initiating mass. This missing mass or mass defect has been converted into energy. Here uranium-235 undergoes nuclear fission after capturing a neutron, it splits into barium-141 and krypton-92, releasing three neutrons and a large amount of energy:
235 U + 1n → 141 Ba + 92 Kr + 3 1n
The newly released neutrons can impact other uranium nuclei and cause them to fission as well, if it could result in a chain reaction, then a nuclear reaction has occurred. This chain reaction is the principle of working of nuclear reactors and manufacture of atomic bombs.
2. Fusion: Nuclear fusion is the kind of nuclear reaction whereby two particles of light atomic number combine to form a heavier atomic number during which energy is also produced. Fusion is the process through which the sun and other stars generate light through a process where hydrogen nuclei combine at very high pressure and temperature to form helium.
The fusion of deuterium and tritium, isotopes of hydrogen, is one of the most researched fusion reactions due to its relatively high energy yield:
2H + 3H → 4He + 1n
This reaction yields a helium nucleus and a neutron besides giving off about 17.6 MeV energy which is way much than most fission reactions. The problem of utilizing fusion energy on Earth is to create and maintain the temperature and pressure that are necessary to begin the fusion process.
Energy Dynamics in Nuclear Reactions
The energy released in nuclear reactions is much greater than in chemical reactions because the binding energy of the nucleons in the nucleus is much greater than the energy that binds electrons in atoms. Binding energy per nucleon (amount of energy required to strip a nucleon off the nucleus on average) also differs from element to element and shows an overall increasing trend with atomic number right up to the iron and a decreasing trend top down the chart. This is why energy can be released by nuclear fission on the one hand, as well as by nuclear fusion on the other hand.
Therefore, it is possible to determine the energy produced by a nuclear reaction in practical terms by using the mass defect and Einstein’s equation E=mc2. This relationship offers the theoretical background in this subject that explains the huge potential of nuclear energy and its destructive force as a weapon and as the potential source of energy production.
NOTE: Thus, nuclear reactions are one of the foundations of nuclear chemistry and they have a gigantic importance for the future energy requirements and medicine. To coordinate such reactions, from predicting them theoretically to implementing them in practice, is still the major task and the pivotal challenge in science.
Explain the different types of radiation
In nuclear chemistry, radiation means energy particles or waves ejected from the nucleus of an unstable atom that is undergoing radioactive decay. There are three principal forms of radiation: Alpha, Beta, and Gamma which have their own characteristics. Gaining familiarity with these types of radiation, their characteristics, how they can be measured, and relative order in decay series is important in various practical uses including medical treatments and energy production.
What is Alpha Radiation?
Alpha radiation contains alpha particles, particles made up of two protons and two neutrons, which are in fact helium nuclei. During radioactive decay, these particles are ejected from the nuclei of extremely heavy atoms, for example, of uranium or radium. Alpha particles are large and they are doubly charged particles. Because of the charge and mass of the particle, alpha particles themselves can only penetrate a sheet of paper or a few centimetres of air deep.
Nonetheless, alpha particles, although sparingly penetrative, possess high ionising capability within a restricted area of penetration, and can thus be damaging to materials it interacts with such as human tissue.
What is Beta Radiation?
Beta radiation is the process by which Beta particles which include high-energy, high speed electrons or positrons are expelled from the nucleus of a particular atom. E beta particles are much smaller in size compared with the alpha particles and carry a single electron charge either negative or positive.
Referred to as beta particles, they are more penetrative compared to alpha particles but can be stopped by a sheet of paper or thin layer of clothing or only 3 millimetres of soft aluminum. Beta radiation marginally ionises and can traverse through living tissue and ionise along the path, which is damaging.
What is Gamma Radiation?
Gamma radiation includes a gamma ray which is a high energy electromagnetic wave which is emitted from the nucleus. Different from alpha and beta radiation, gamma rays are waves, not particles, and therefore have no mass and/or charge. They have highly enhanced penetration capability and can easily get through several centimeters of lead or meters of concrete. The least penetrating of the three types of radiation is gamma rays, but due to its great penetration capacity, it causes an internal and external health hazard.
Understanding safety and risks in nuclear chemistry
There are certain constraints within nuclear chemistry especially concerning safety and regulation of radiation and radioactive materials. Proper handling, usage, and disposal of nuclear material is a complex process that requires precautionary measures, sophisticated national and international laws, and keen adherence to internationally approved standards and norms. This article will briefly discuss basic radiation protection, nuclear power generation, positive and negative measures, and disposal of radioactive waste.
Safety measures due to radiation: Safety in environments where radiation is used or produced revolves around three fundamental principles: Time, Distance, and Shielding. The exposure time near the radioactive sources should be reduced to the barest minimum, avoid staking close to the sources, and putting on protective shielding materials.
Management of wastes and safety disposal: Control and disposal of nuclear waste are some of the major issues of nuclear safety. They include safe management, treatment, and disposal of Nuclear waste materials. Low-level waste (LLW), Intermediate Level waste (ILW), and High-Level waste (HLW) are nuclear wastes that have their own disposal methods.
NOTE: Nuclear chemistry is an important aspect that is critical in the promotion of health and safety measures and ensures the sustainability of nuclear technology as one of the most vital energy resources in the contemporary world.
To ensure a safe future for nuclear technology, various effective radiation safety measures need to be taken like robust regulatory frameworks, and meticulous waste management and disposal strategies to manage the risks associated with nuclear materials.






