September 01, 2024
Particles and waves: The central mystery of quantum mechanics
One of the most astonishing discoveries in the realm of physics is the realization that everything in the universe, from light to electrons to atoms, behaves both like a particle and a wave. This dual nature forms the cornerstone of quantum mechanics and underpins many of its seemingly bizarre phenomena, such as Schrodinger's Cat, the probabilistic nature of particles, and quantum entanglement.
The Curious Case of Light: A Wave or a Particle?
At first glance, the idea of something being both a particle and a wave seems counterintuitive. After all, waves in water and particles of rock appear entirely distinct. Yet, physicists didn’t arbitrarily combine these concepts. Instead, they were led to this dual nature through a series of incremental discoveries, piecing together evidence like a complex puzzle.
The first serious suggestion of the dual nature of light came from Albert Einstein in 1905, building on earlier ideas from Max Planck. Planck had attempted to explain the colors of light emitted by hot objects, such as a light bulb filament, but his explanation required a radical assumption.
He proposed that these objects were made up of oscillators that could only emit light in discrete chunks, or quanta, of energy dependent on the light's frequency. Though Planck was uneasy with this concept, Einstein expanded on it, proposing that light itself, traditionally understood as a wave, also behaved as a stream of particles—later known as photons.
Einstein’s theory, revolutionary in its implications, explained phenomena like the photoelectric effect, where light shining on a metal surface ejects electrons. Even those who initially resisted the idea were eventually convinced by the overwhelming evidence.
Rutherford's Atom: A Revolutionary Model with a Flaw
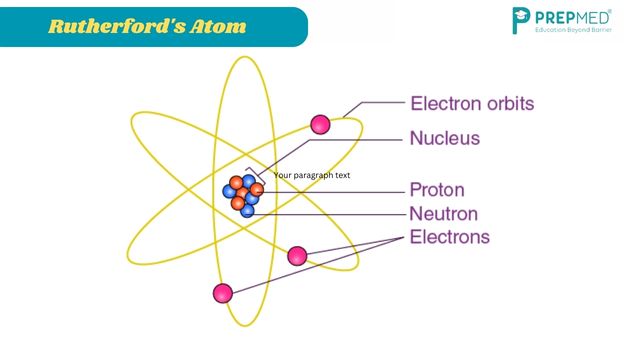
Meanwhile, in England, another puzzle piece was added by Ernest Rutherford. In 1909, Rutherford’s associates, Ernest Marsden and Hans Geiger, conducted an experiment that involved shooting alpha particles at gold atoms. They were surprised to find that some particles bounced straight back, leading Rutherford to conclude that most of the atom’s mass was concentrated in a tiny nucleus, a model now famously represented as electrons orbiting a central nucleus like planets around the sun.
However, Rutherford’s model faced a critical problem: according to classical physics, electrons in orbit should continuously emit radiation, losing energy and eventually spiraling into the nucleus. This contradicted the observed stability of atoms, suggesting that something fundamental was missing from the classical understanding of physics.
Bohr’s Quantum Leap: A New Model for Atoms
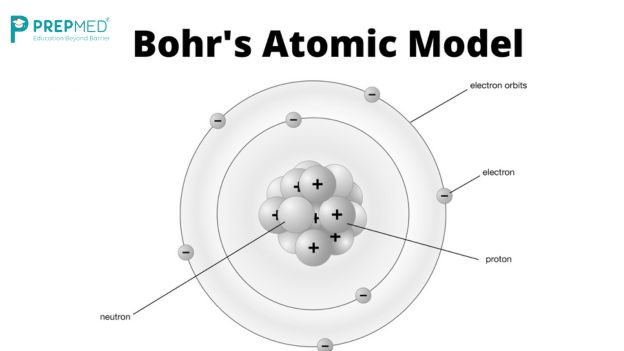
Niels Bohr, a Danish theoretical physicist working with Rutherford, proposed a solution. He suggested that electrons occupy certain special orbits where they do not emit radiation. Atoms, Bohr proposed, absorb or emit light only when electrons jump between these orbits, with the frequency of the emitted light directly related to the energy difference between the orbits. This explanation not only resolved the issues with Rutherford’s model but also accounted for the specific colors of light emitted by different elements.
However, Bohr’s model introduced a new mystery: why were these orbits special? The answer came from an unexpected source.
Louis de Broglie: The Electron as a Wave
Louis de Broglie, a French PhD student, made a groundbreaking suggestion that completed the puzzle. If light, traditionally seen as a wave, could behave like a particle, then perhaps electrons, known to be particles, could behave like waves. This wave behavior could then explain why electrons occupied specific orbits in Bohr’s model, as these orbits corresponded to the wavelengths of the electron waves.
Once de Broglie’s idea was introduced, scientists quickly sought evidence for the wave nature of electrons. Within a few years, experiments in the United States and the United Kingdom confirmed this wave behavior. Today, one of the clearest demonstrations involves shooting single electrons at a barrier with slits cut into it. While each electron is detected at a specific place and time, like a particle, repeating the experiment reveals a pattern characteristic of wave interference, unmistakably demonstrating the electron’s wave-like nature.
The Legacy of Particle-Wave Duality
The discovery that particles behave like waves, and vice versa, stands as one of the most profound and perplexing discoveries in physics. Richard Feynman, one of the greatest physicists of the 20th century, famously described this as the central mystery of quantum mechanics. Everything else in the field, from the uncertainty principle to quantum entanglement, follows from this dual nature of matter and light, much like the pieces of a puzzle falling into place.
The dual nature of particles and waves not only reshaped our understanding of the physical world but also laid the foundation for modern quantum mechanics, leading to technologies that define our age, from semiconductors to quantum computers. As we continue to explore the quantum realm, the particle-wave duality remains at the heart of the mysteries yet to be unraveled.
For more details on PrepMed and to find the nearest coaching center, visit PrepMed’s official website
Follow Us - on Facebook /Instagram/ YouTube