February 07, 2025
Exploring Chemical Kinetics: Rate Laws and Mechanisms
Studying chemical kinetics reveals essential knowledge about the mechanisms and rate laws that control chemical reactions. Physical chemistry's critical subdivision, called chemical kinetics, studies how fast chemical reactions proceed along with the factors that affect these reaction rates. The research reveals how the speed of reactions depends on molecular collisions between reactants as well as reactant concentrations, and both temperature conditions and the presence of a catalyst. The fundamental components of chemical kinetics include rate laws, which express the relationship between reaction speed and reactant amount, while mechanisms illustrate the sequence of elementary reactions that build the overall transformation.
Studying chemical kinetics reveals essential knowledge about the mechanisms and rate laws that control chemical reactions. Chemical kinetics studies how fast chemical reactions proceed, along with the factors that affect these reaction rates.
The research reveals how the speed of reactions depends on molecular collisions between reactants as well as reactant concentration, temperature, and the presence of a catalyst.
Chemical Kinetics: Fundamental Components
The fundamental components of chemical kinetics include rate laws, which express the relationship between reaction speed and reactant amounts, while mechanisms illustrate the sequence of elementary reactions that build the overall transformation.
Scientific understanding of fundamental principles allows researchers to predict reaction outcomes while enhancing industrial processes and creating pharmaceutical products as well as resolving environmental problems.
Chemical Kinetics: Overview
The study of chemistry class 12 chemical kinetics deepens our understanding of reaction dynamics while enabling technological advancements through diverse scientific applications, thus establishing its fundamental role in present-day chemistry. This introduction to chemical kinetics will provide a deeper look into rate laws, reaction mechanisms, and the principles that govern how and why chemical reactions proceed at different speeds.
What do you mean by “Chemical Kinetics?”
The field of chemistry that emphasizes studying the rates of various chemical reactions and the factors that influence how quickly or slowly a reaction occurs is known as Chemical Kinetics.
Chemical kinetics deals with understanding the rate of reactions, how conditions (like temperature, concentration, and pressure) affect the reaction rate, and the mechanisms or steps by which reactions take place at the molecular level.
Define the rate of reaction.
The rate of reaction refers to the speed at which reactants are converted into products in a chemical reaction. It is usually expressed as the change in concentration of reactants or products per unit of time. For example, it can be measured in terms of how much the concentration of a reactant decreases or the concentration of a product increases over a specific period.
What are the factors influencing the rate of reactions?
Several factors influence the rate of reaction:
- Concentration of Reactants: Generally, the higher the concentration of reactants, the faster the reaction. The availability of more particles to collide with each other results in faster reaction rates.
- Temperature: An increase in temperature normally causes reactions to move more quickly. The increased temperature gives reacting molecules greater energy that enables them to collide a greater number of times with increased kinetic energy, thus promoting successful reactions.
- Surface Area of Reactants: The reaction rate becomes faster when reactants are divided into smaller components, which increases their surface area. An increased number of particles becomes accessible for collision, thus speeding up reaction rates.
- Presence of a Catalyst: Catalysts in chemical reactions function by decreasing activation energy so the reaction begins more quickly yet they stay intact during the process. Reactants transform into products more efficiently because of this effect.
- Pressure: The rate of gas reactions increases when pressure levels rise. The compression of gas molecules sparks closer interaction, which boosts the number of molecular collisions.
Unraveling Rate Laws: Understanding Reaction Speed
In chemical kinetics, rate laws are mathematical expressions that describe the relationship between the concentration of reactants and the rate at which a reaction occurs. Understanding rate laws is essential for predicting how changes in reactant concentration, temperature, or other factors affect the speed of a reaction.
A rate law typically takes the form:
Rate = k [A]^m [B]^n
Where:
- The rate is the reaction rate.
- k is the rate constant, which is a proportionality constant that depends on temperature and the nature of the reaction.
- [A] and [B] are the concentrations of the reactants.
- m and n are the orders of the reaction with respect to each reactant, which indicate how the concentration of each reactant affects the rate.
Key Concepts in Rate Laws:
- Reaction Order: The exponents in the rate law indicate the reaction order with respect to each reactant. For example, if the rate law is first order with respect to reactant A (m = 1), then doubling the concentration of A will double the reaction rate. If m = 2, doubling A will quadruple the rate.
- Rate Constant (k): The rate constant reflects how easily a reaction occurs at a given temperature. It can be determined experimentally and is specific to a particular reaction.
- Overall Order of the Reaction: The sum of the exponents in the rate law gives the overall order of the reaction. A reaction may be first-order, second-order, or higher, depending on the sum of the individual reaction orders.
Define half half-life of a first-order reaction
For a first-order reaction, the half-life (t ½ ) is constant and independent of the initial concentration of the reactant. The relationship for the half-life of a first-order reaction is given by the formula:
t½ =ln2/k
Where:
- t½is the half-life,
- k is the rate constant for the reaction,
- ln 2 is the natural logarithm of 2 (approximately 0.693).
Since the half-life for a first-order reaction depends only on the rate constant k, it is the same throughout the reaction process.
Define half-life of an order reaction
For a zero-order reaction, the half-life t½ depends on the initial concentration of the reactant. The relationship for the half-life of a zero-order reaction is given by the formula:
t½ =[A]0/2k
Where:
- t½ is the half-life.
- [A]0 is the initial concentration of the reactant,
- K is the rate constant for the reaction.
In a half-life of a zero-order reaction, the half-life decreases as the initial concentration of the reactant decreases, unlike in a first-order reaction, where the half-life is independent of concentration.
Unit of zero-order reaction
Since the rate of the reaction has units of concentration per time (e.g., mol/L⋅s), the unit for a zero-order reaction of the rate constant k is:
k=mol/L⋅s
Thus, the unit of the rate constant k for a zero-order reaction is mol/L·s.
Unit of second-order reaction
The unit for a second-order reaction of the rate constant k:
L⋅mol−1⋅s−1
The Role of Concentration in Chemical Reaction Rates
Chemical reaction speed depends critically on the amount of reactants present in the solution. In chemical kinetics, the higher the concentration of reactants, the faster the reaction rate. The higher reactant concentration in the system creates additional particle density that results in elevated molecular collision frequency.
How Concentration Affects Reaction Rate:
- More Collisions, Faster Reaction
Higher reactant concentration results in increased numbers of molecules or ions within a defined volume. The greater number of particles in solution leads to increased collision frequency, resulting in more successful reactions. More collisions per unit of time typically result in a higher reaction rate.
- Effect on Reaction Rate Law
In rate laws of chemical reactions, the concentration of reactants plays a vital role since the reaction rate always depends on the concentration levels of certain reactants elevated to certain powers(reaction order). A first-order reaction shows a proportional relationship between its rate and the concentration of the single reactant present. The rate of a second-order reaction depends directly on the square of the concentration of its reactants.
- Saturation and Limiting Reactants
An increased concentration typically boosts reaction speed, but after reaching a certain threshold, additional reactant addition fails to accelerate the process. A reaction typically reaches saturation when it becomes too slow because of additional limiting factors like temperature, pressure or the presence of a catalyst.
Factors Affecting Reaction Rates
The reaction speed in chemical kinetics depends primarily on temperature, pressure, and other factors.
The rate at which chemical reactions take place depends on various influencing factors. Common reaction-rate determining factors in chemical kinetics include temperature, pressure, concentration, surface area, and the usage of catalysts.
1. Temperature
- Temperature elevation typically speeds up the pace at which chemical reactions take place. Higher temperatures provide additional energy to reacting molecules which leads to increased speed of movement and higher collision frequency between molecules. Strong collisions between molecules accelerate chemical reaction success.
2. Pressure (For Gaseous Reactions)
- Effect on Reaction Rate: For reactions involving gases, increasing the pressure usually increases the reaction rate. When pressure is increased, the gas molecules are compressed into a smaller volume, causing more frequent collisions between molecules.
3. Concentration of Reactants
- Effect on Reaction Rate: As previously mentioned, higher concentrations of reactants lead to more collisions between particles, which generally increase the reaction rate. More reactant molecules or ions in a given volume increase the probability of successful collisions.
4. Surface Area of Reactants
- Effect on Reaction Rate: The larger the surface area of a solid reactant, the faster the reaction will proceed. This is because a greater surface area allows more particles of the solid to come into contact with the other reactant, leading to more collisions and a higher reaction rate.
5. Presence of a Catalyst
- Effect on Reaction Rate: A catalyst is a substance that increases the reaction rate without being consumed in the reaction. Catalysts work by lowering the activation energy required for the reaction, allowing more molecules to react successfully.
6. Nature of Reactants
- Effect on Reaction Rate: In chemical kinetics, the chemical nature of the reactants also plays a significant role in the reaction rate. Some reactions, such as those involving ionic compounds or gases, tend to occur much more rapidly than others, such as those involving covalent bonds or solids.
Let’s have a look at one of the very important MCQ from the chapter Chemical Kinetics:
Question 1: In a zero-order reaction, for every 10°C rise in temperature, the rate doubles. If the temperature is increased from 10∘C to 100∘C, the rate of the reaction will become
- 256 times
- 512 times
- 64 times
- 128 times
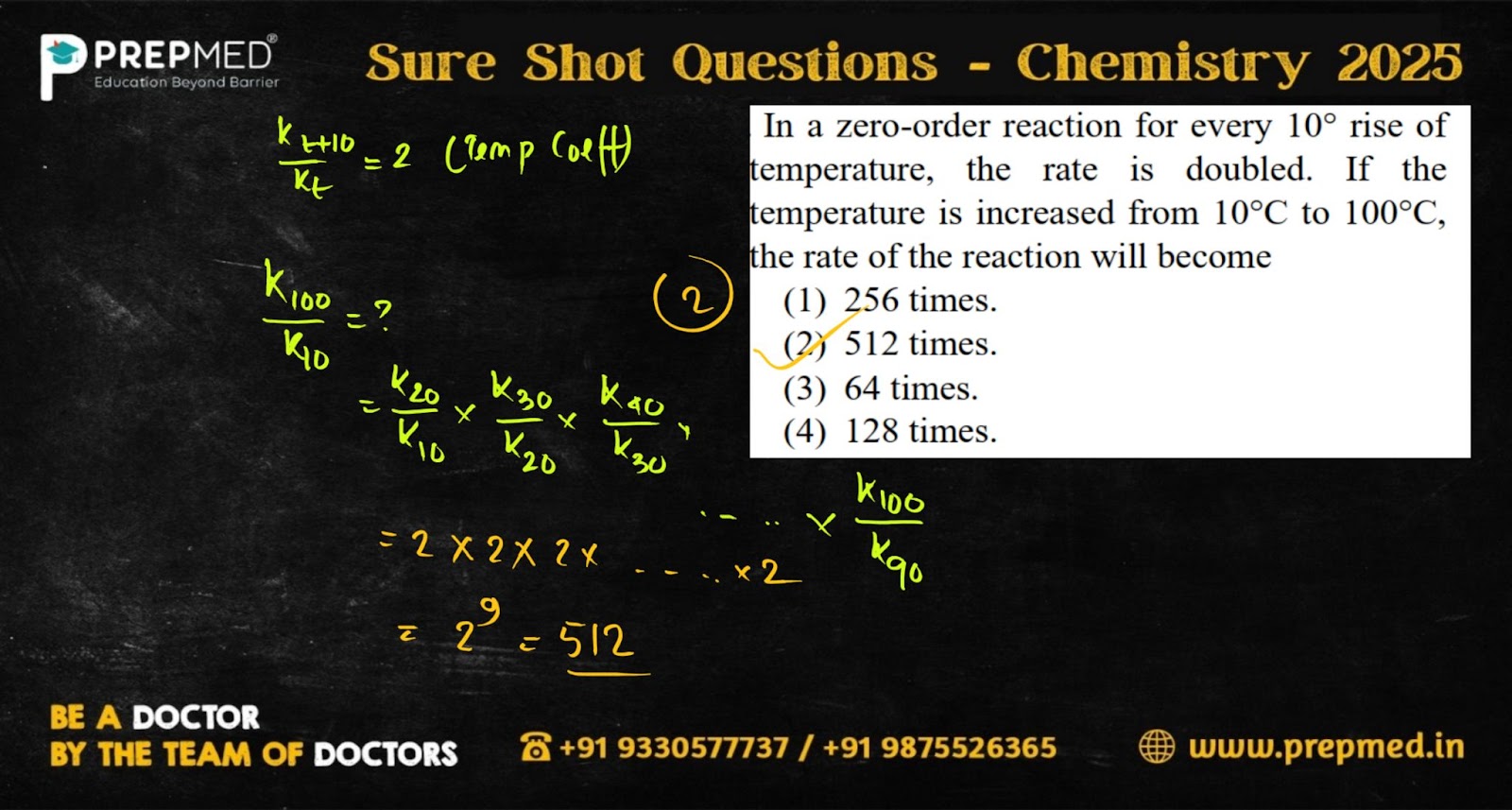
The correct answer is Option 2
Question 2:
- D>A>B>C
- A>B>C>D
- C>A>B>D
- D>B>A>C
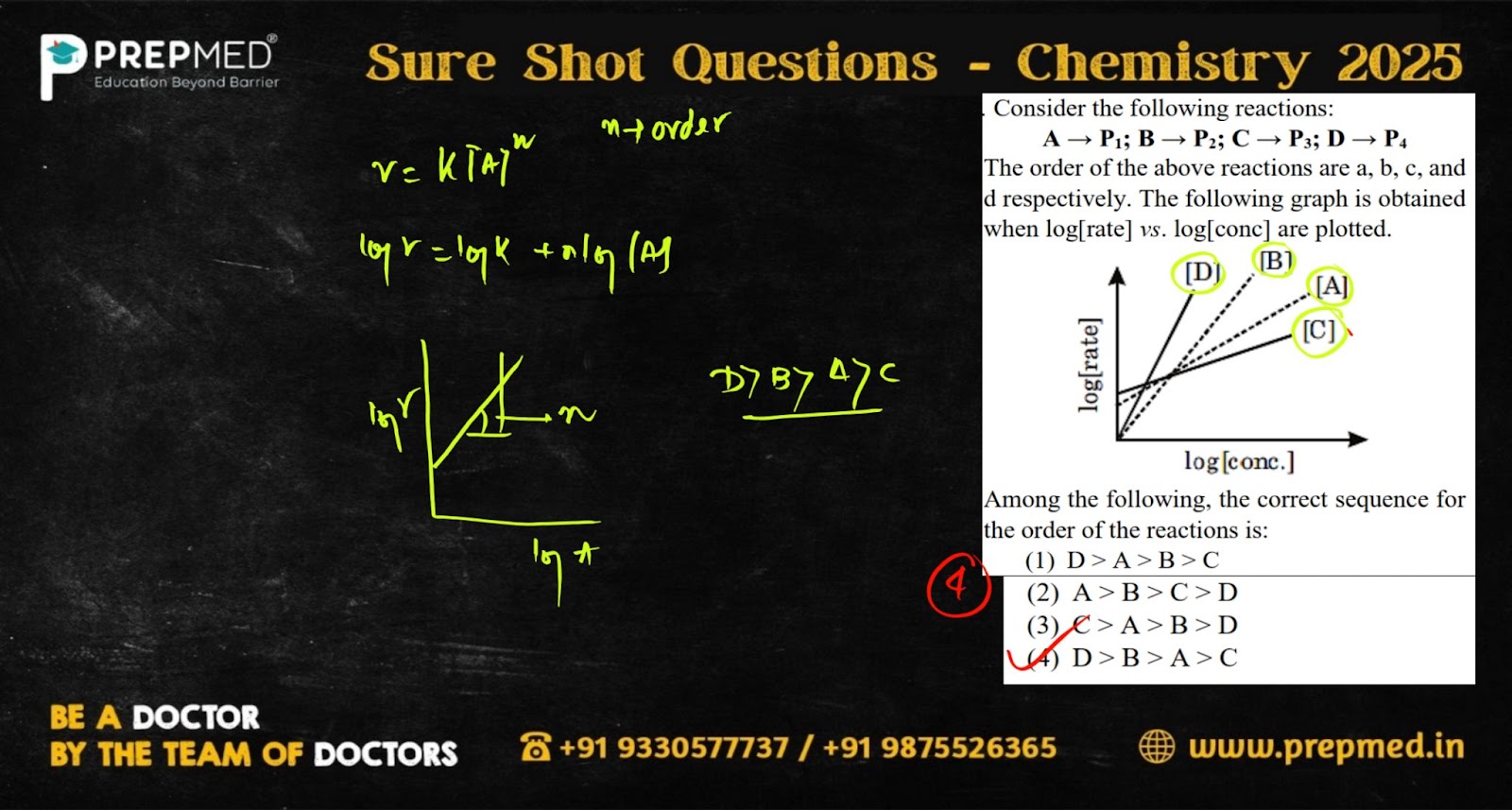
The correct answer is Option 4
Conclusion:
Exploring chemical kinetics, particularly rate laws and mechanisms, provides essential insights into how reactions occur and their rates. Rate laws, which relate reaction rates to reactant concentrations, offer predictive power about the speed of reactions. Understanding the different orders of reactions —zero, first, and second—helps in determining how factors like concentration and temperature influence the reaction rate.
FAQs:
- What is the rate law in chemical kinetics?
The rate law in chemical kinetics expresses the relationship between the rate of a reaction and the concentration of its reactants. It is determined experimentally and shows how the rate depends on the concentration of reactants raised to specific powers (which are the reaction orders).
- How do reaction orders affect the rate of a reaction?
The reaction order indicates how the concentration of a reactant influences the reaction rate in chemical kinetics. For example, in a first-order reaction, the rate is directly proportional to the reactant concentration, while for a second-order reaction, the rate is proportional to the square of the concentration.
- What is the significance of reaction mechanisms in chemical kinetics?
Reaction mechanisms describe the step-by-step sequence of elementary reactions that lead to the overall reaction. Understanding mechanisms in chemical kinetics helps identify intermediates, determine rate-determining steps, and optimize reaction conditions for efficiency.






